|
Abstract |
Abstracts |
|
2.1 Malaria and culicids |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2.1.1 Course of the parasitosis Malaria appears in three clinical forms or nosodemes: Malaria tertiana (Plasmodium vivax, P. ovale), M. quartana (P. malariae) and M. tropica (P. falciparum). Originally, M. tertiana and M. quartana were found in the northern temperate zones,too, whereas M. tropica is endemic in the tropics only. Plasmodia belong to the Sporozoa (syn. Apicomplexa). They occur in man and many primates as well as in bats (Chiroptera) and rodents, but not in canids (dogs), suids (porks), ovines (sheep) and bovins (cattle). Birds and reptiles also harbour Plasmodia as parasites. Culicids, mosquitoes of the genus Anopheles tranfer the sporozoites of the Plasmodia of mammals . Within the first seconds after its infective bite, the sporozoites leave the circulating blood in order to enter the parenchymatous cells of the liver. Here they undergo a livercell-parasitic phase in human malaria. This is limited from 13 to 15 divisions, depending on the plasmodium species. In P. vivax and P. ovale additionally hypnozoites are formed, which divide later on, causing febrile relapses (fig. 2.1, page 12). The following erythrocyte-parasitic phase starts synchronically when the merozoites are being released all at the same time from the meronts (schizonts) of the liver. In the circulating blood the first cycles of merogonia (schizogonia) are completed almost without losses. As soon as a density of infected RBC (red blood cells) is reached in the circulating blood, essential to continue the parasitosis, the body reacts with a fever, which drastically reduces the successful invasion rate of merozoits into new RBCs. During this critical phase, especially in a first infection with P. falciparum, the parasitaemia may exeed the infection carrying capacity of its host and thus quickly lead to its death, particularly in infants. During the following chronical phase the level of the parasitaemia is regulated by an immunological feed-back mechanism (fig. 2.2, page 15). The cycles of the merogonia in the circulating blood end after some while on its own. Each species of Plasmodium is seen to have a specific life expectancy, identified by the mean number of fever cycles or the duration of an untreated infection, respectively (fig. 2.5, page 19). A regulation of parasite density in the circulating blood mediated by antibodies, leads to an almost constant level of parasitised RBCs (fig. 2.2, page 15). Depending upon the number of merozoites per meront, the multiplicative factor, a significant amount of foreign protein infiltrates permanently into the host’s body. At the same time, a short term excess of parasite antigen, the smoke screen, protects the merozoites against circulating antibodies until they have entered a new RBC. The long-time stabilisation of the maintenance phase is probably based on a dynamic modulation of the immune response by the plasmodium. Consequent upon this, no protective immunity arises, that means, the spontaneous self-healing is not the result of an immune response. The trophozoites periodically create sexually differentiated gamonts (gametocytes), of which microgametes swarm inside of the gut of the mosquito within minutes in order to fertilise the macrogametes. The zygota (ookinete) penetrates the peritrophic membrane of the mosquito and develops into an oocyst in the basal lamina of the intestinal epithelium, Thereafter, following a meiosis and 12 divisions, about 4000 sporozoites (P. falciparum) are produced, which enter the salivary cells of the mosquito via the hemolymph (fig. 2.9, page 24). As a rule, they are subsequently transmitted by the female mosquito during the next but one, the third, blood meal. Intracellular parasitism accompanied by extremely rapid changing of the host cell, self-control of propagation, synchronisation of stages, permanent inflow of Plasmodium antigenes combined with positive and negative feed back mechanisms, all guarantee a long lasting balance between the Plasmodium and its host. These factors result in a maximal prevalence (infection rate) of the host population combined with a limited lethality. The so-called chemoprophylaxis is actually a long-term medical treatment on the suspicion of infection. 2.1.2 Biology of Culicids The mosquitoes or Culicids are two-winged flies (Diptera), belonging to the suborder Nematocera. Culicids breed in still water. Their larvae and pupae develop only in still water, though of remarkable different types (fig. 2.7, page 21). The hydro-chemical conditions of such breeding waters are specific and determine the geographical and seasonal distribution of genera, species and cytotaxonomic types (see 2.2,.2 p. 43). The finding of the sexes – male and female - is mediated by acoustic stimuli. During swarming, males recognise passing females via specifically differentiated antennae as a result of their species-specific wing beat frequencies . The females orientate to their blood hosts by a series of stimuli. Initially, they detect host odours which cause the females to take off. Once in flight, they orientate by a process of negative anemotaxis. Both reactions are repeated several times. Upon reaching the proximity of the blood host, the increasing concentrations of CO2 lead to its closing attraction. Eventually, location and settling on the host are directly the result of the host’s body warmth and the humidity of its skin, which dominate as attractive stimuli (fig. 2.7, page 21). Female mosquitoes are capillary as well as pool feeders. The forces for penetration of the host’s skin are created using the flexibility of the chitinous stiletts (fig. 2.8, page 23). During biting, saliva is repeatedly injected into the host blood stream. As a consequence of this feeding, blood is directly taken up into the intestinal gut , whereas nectar first enters the crop. The microclimate of the specific resting places used for digestion following the blood meal is also important for the development of the parasites, which are only imbibed occasionally. In fact, the geographical distribution of a Plasmodium may be more limited than that of its insect host. The age of the females can be determined by the number of gonotrophic cycles it has undergone (fig. 6.7, page 250). Under natural conditions in the field, Plasmodia are as mentioned earlier usually transmitted after the, the third feeding after the gamonts had been taken up. 2.1.3 Quantitative epidemiology The population potentially threatened by the parasitosis is the population at risk.-.The prevalence describes the extent of established cases of the disease, the incidence the number of new cases of an outbreak of the disease within a certain space of time and lethality is the rate of cases of death related to the diseased ones. The prevalence of gamont carrying patients multiplied by the density of the gamonts in their blood form the infective reservoir (medical aspect). The population dynamics of the parasite, here of the Plasmodium, indicates its quantitative changes within a time frame (biological aspect). For its assessment entomological, parasitological and clinical data are used. Under stable endemic conditions the inoculation rate (infective bites per man and day) and the vectorial capacity (potentially new infections per gamont carrier and day) are in equilibrium (fig. 2.10, page 27). The population of a parasite comprises the sum of the individual parasite loads. The distribution of the parasite in the host population is negative-binomial. In a stable endemia the influx (acquisition) and the outflow (removal) are equilibrated in the whole population and result in the turnover. Its absolute measure is the change of masses. The suitability of the vectors for the transmission of malaria is estimated by the vector efficiency. It comprises the anthropophilia of the mosquito, the mean life expectancy (age) of the infected mosquito, its susceptibility, the feeding rate and duration and the mean load of sporozoites. All these values are endogenically determined, i.e. independent from the population density of the vectors and their hosts. Endogenic factors of the Plasmodium compensate for seasonal changes, for the population density of the vectors as well as for the susceptibility of the warm-blooded host (man) and the change of stages in the homoiothermic animal. The prevalence of malaria generally decreases with increasing age of the human host. In certain regions it may vary considerably with the saisons, as can be seen with the density of trophozoites and gamonts. In this case both measures are highest in the youngest age groups and lowest in the most upper ones.However, the prevalence and density of gamonts increases manyfold particularly in higher age groups at the beginning of the rainy season,. In this way the ageing drift of the plasmodium is compensated for and the transmission favoured with increasing density of the vectors (fig. 2.11, page 31). A seasonal shifting directs the merogonia cycles in the blood to those with and without gamonts formation (flux diagram fig. 2.12, page 32). At the same time, with decreasing exposition, e.g. during the dry season, the susceptibility of man increases again. Together with a large proportion of gamont carriers at the beginning of the rainy season it triggers a cascade effect, which quickly boosts the transmission. The transmission by a vector evolved to require an adaptation to two hosts of different phyla. With increasing complexity of the life cycle the extended possibilities for feed back mechanisms offer the parasite better chances of survival. Heteroxenous parasites are extremely successful. Their basic case reproduction rate (Ro) is severalfold higher than those of bacterial or viral infections. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Seasonal compensation of gamogonia in Plasmodium sp. and susceptibility in human hosts. Dates from Molyneaux and Gramiccia 1980. 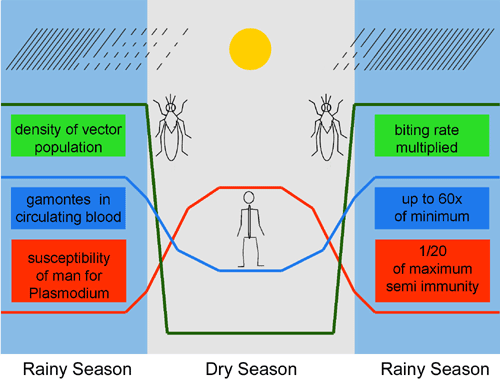 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
start |